Abstract
Background Genetic reports of patients with severe AA (SAA) often identify heterozygous variants of unknown significance in recessive genes associated with inherited bone marrow failure syndromes (IBMFS), such as Fanconi anemia (FA) and telomere biology diseases (TBD). More recently, heterozygous SAMD9 and SAMD9L (SAMD9/9L) variants have also been reported in children and young adults with BMF, but whether all variants are gain-of-function (GoF) and pathogenic is unknown. In a large cohort of SAA patients, we investigated the prevalence of heterozygous variants in SAMD9/9L and FA/TBD genes and correlated them to disease characteristics and clinical outcomes.
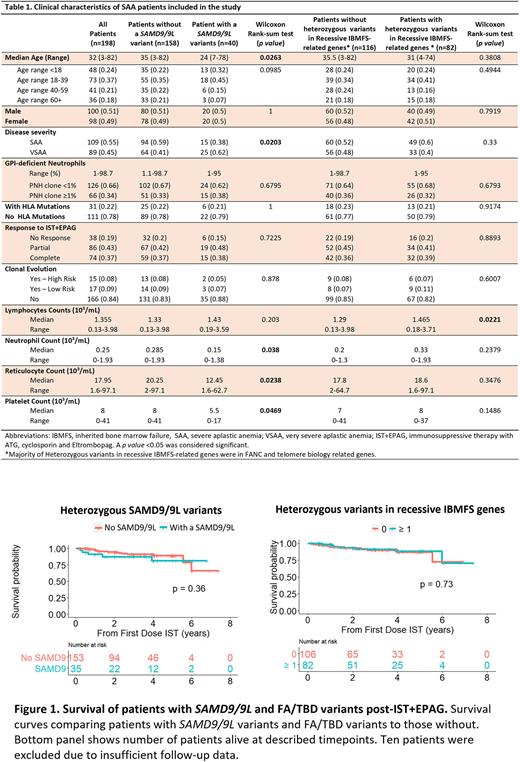
Methods SAA patients (n=198; median age [range]: 32 [3-82]) enrolled on a phase II trial for treatment with horse anti-thymocyte globulin, cyclosporine, and eltrombopag (NCT01623167) at the National Institutes of Health from 2012 - 2022 were included (Table 1). All patients were screened for germline mutations in IBMFS related genes. SAMD9/9L variants were segregated by rarity according to frequency in the gnomAD database as common (<0.1% in overall population, including variants with maximum population frequency [MaxPopFreq] >1%), rare (MaxPopFreq <0.1%), very rare (MaxPopFreq <0.01%), ultra-rare (MaxPopFreq <0.005%), and novel (absent in gnomAD).
Results We first investigated whether patients had an undiagnosed SAMD9/9L disorder. Although 40/198 patients (20%) had a heterozygous SAMD9/9L variant, including 8 (20%) with 2 variants, these were mostly classed as common (73%; n=35), followed by rare, very rare, ultra-rare and novel variants seen in 17% (n=8), 4% (n=2), 2% (n=1), and 4% (n=2), respectively. Most variants were missense (n=39; 81%), followed by nonsense (n=6; 13%) and frameshift mutations (n=3; 6%). Except for ultra-rare and novel, SAMD9/9L often co-occurred with typical features of immune AA (6p-LOH, PNH clones, HLA and PIGA mutations). None of the 40 patients with germline SAMD9/9L variants had primary somatic genetic reversions (SGR) such as somatic loss of function (LoF) mutations (VAF>0.5%) in SAMD9/9L or UPD7q. Since GoF SAMD9/9L variants are located on chr7 and they associate with development of monosomy 7, we investigated whether SAMD9/9L variants in our cohort were associated with clonal evolution after treatment. However, only 2/40 patients (5%) with chr7 abnormalities had these variants, both common.
Patients with SAMD9/9L variants, both common and rare, were significantly younger (35 years vs. 24 years, p=0.026) and more frequently had VSAA (defined as absolute neutrophil count <0.2 K/uL) than those without these variants (62% vs. 41%, p=0.02); they had lower median neutrophil counts (p=0.038), reticulocyte counts (p=0.024), and platelet counts (p=0.047). The presence of rarer (MaxPopFreq<0.1%; n=11) or common SAMD9/9L variants (n=29) did not impact disease severity, or correlate with molecular characteristics of immune AA, response to immunosuppressive therapy, and overall survival (Figure 1).
In addition to SAMD9/9L variants, 82 patients (41%) had 1 or more heterozygous variants in FA/TBD, but these were not associated with disease severity or with clinical outcomes, even when co-occurring with SAMD9/9L variants. Twenty five patients (13%) had co-occurring heterozygous mutations in both SAMD9/9L and FA/TBD genes, but these were not correlated with disease severity or response to therapy when compared to patients without co-occurring mutations.
Conclusions As opposed to classical SAMD9/9L disorders in pediatric cohorts, SAMD9/9L variants found in our primarily adult patients with SAA are not disease-causing germline GoF mutations, even the rarest variants. Most patients with variants responded to immunosuppressive therapy, and the less rare variants were associated with molecular features of immune AA but not SGR, both findings suggestive of immune pathophysiology. Interpretation of genetic reports, including SAMD9/9L, in adults appears to be different from mostly pediatric cohorts with SAMD9/9L disorders. Heterozygous SAMD9/9L variants do affect AA severity but are not predictive of response to therapy or prognosis. FA/TBD heterozygous variants did not impact disease characteristics or outcomes. We did not observe a higher rate of clonal evolution in patients with SAMD9/9L after EPAG treatment compared to patients carrying FA/TBD variants and others without variants.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal